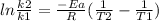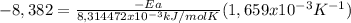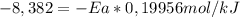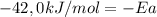Chemistry, 16.12.2019 21:31 krandall232
Areaction is followed and found to have a rate constant of 3.36 × 104 m-1s-1 at 344 k and a rate constant of 7.69 m-1s-1 at 219 k. determine the activation energy for this reaction.23.8 kj/mol11.5 kj/mol12.5 kj/mol42.0 kj/mol58.2 kj/mol

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:50
An aqueous solution of potassium hydroxide is standardized by titration with a 0.194 m solution of hydrobromic acid. if 25.2 ml of base are required to neutralize 24.2 ml of the acid, what is the molarity of the potassium hydroxide solution? m potassium hydroxide
Answers: 2

Chemistry, 22.06.2019 03:40
In an effort to address concerns about global warming, a power plant in portland,oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1

Chemistry, 22.06.2019 10:00
Drug abuse will not lead to physical and psychological dependence. true or false ?
Answers: 2

You know the right answer?
Areaction is followed and found to have a rate constant of 3.36 × 104 m-1s-1 at 344 k and a rate con...
Questions



Computers and Technology, 15.11.2019 23:31

Biology, 15.11.2019 23:31


Mathematics, 15.11.2019 23:31

History, 15.11.2019 23:31

Mathematics, 15.11.2019 23:31

History, 15.11.2019 23:31



Chemistry, 15.11.2019 23:31



English, 15.11.2019 23:31


Health, 15.11.2019 23:31


History, 15.11.2019 23:31

Social Studies, 15.11.2019 23:31