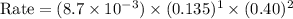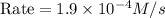Chemistry, 16.12.2019 19:31 pearpeaerrr1993
Consider the following reaction between mercury(ii) chloride and oxalate ion: 2hgcl2(aq)+c2o2−4(aq)→2cl−(aq)+2co2 (g)+hg2cl2(s) the initial rate of this reaction was determined for several concentrations of hgcl2 and c2o2−4, and the following rate data were obtained for the rate of disappearance of c2o2−4:

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1
You know the right answer?
Consider the following reaction between mercury(ii) chloride and oxalate ion: 2hgcl2(aq)+c2o2−4(aq)...
Questions

Mathematics, 10.12.2021 18:10





Geography, 10.12.2021 18:10

English, 10.12.2021 18:10





History, 10.12.2021 18:10




Mathematics, 10.12.2021 18:10




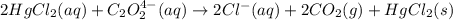
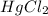 and
and 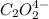 , and the following rate data were obtained for the rate of disappearance of
, and the following rate data were obtained for the rate of disappearance of 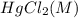
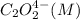 Rate (M/s)
Rate (M/s)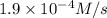
![\text{Rate}=k[HgCl_2]^a[C_2O_2^{4-}]^b](/tpl/images/0420/9839/2a902.png)
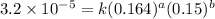 ....(1)
....(1)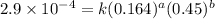 ....(2)
....(2)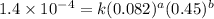 ....(3)
....(3)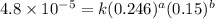 ....(4)
....(4)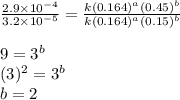
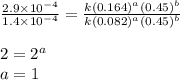
![\text{Rate}=k[HgCl_2]^1[C_2O_2^{4-}]^2](/tpl/images/0420/9839/2292e.png)
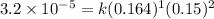
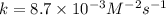
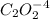 is 0.40 M.
is 0.40 M.