
Alab scientist cools a liquid sample of water (2.6 kg) at 0.00°c to -192°c. the water turns to ice as this temperature change occurs. how much heat is released during this process? [for water, lf = 334 kj/kg and lv = 2257 kj/kg. the specific heat for ice is 2050 j/(kg·k)]. report your answer in kj. (round your answer to a whole number - no decimal places)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:40
Asingle atom of an element has 21 neutrons, 20 electrons, and 20 protons. which element is it? ok o z
Answers: 1

Chemistry, 22.06.2019 17:50
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2

Chemistry, 22.06.2019 21:00
Which property of water causes water drops to bead on a freshly waxed car?
Answers: 2

Chemistry, 23.06.2019 00:00
Which is true about metals used for jewelry, such as platinum and gold? a. they have low flammability. b. they have low reactivity. c. they have high flammability. d. they have high reactivity.
Answers: 1
You know the right answer?
Alab scientist cools a liquid sample of water (2.6 kg) at 0.00°c to -192°c. the water turns to ice a...
Questions






Biology, 14.01.2021 05:40


Mathematics, 14.01.2021 05:40

Mathematics, 14.01.2021 05:40




Mathematics, 14.01.2021 05:40

Mathematics, 14.01.2021 05:40



Chemistry, 14.01.2021 05:40

Mathematics, 14.01.2021 05:40


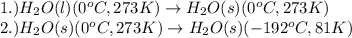
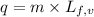 ......(1)
......(1)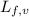 = latent heat of fusion or vaporization
= latent heat of fusion or vaporization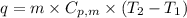 .......(1)
.......(1)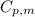 = specific heat capacity of medium
= specific heat capacity of medium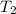 = final temperature
= final temperature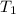 = initial temperature
= initial temperature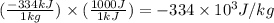
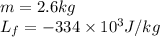
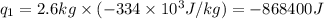
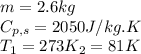
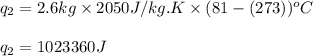
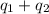
![[-868400+(-1023360)]J=-1891760J=-1891.76kJ\approx -1892kJ](/tpl/images/0420/8409/e23bd.png)


