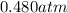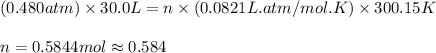Chemistry, 16.12.2019 18:31 Thejollyhellhound20
Carbon dioxide gas is collected at 27.0(degrees)c in an evacuated flask with a measured volume of 30.0l. when all the gas has been collected, the pressure in the flask is measured to be 0.480atm.
calculate the mass and number of moles of carbon dioxide gas that were collected. round your answer to 3 significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 00:30
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1


Chemistry, 23.06.2019 02:50
Select the correct location on the image identify the element that humans need to breathe. 2015 er r ights reserved
Answers: 3

Chemistry, 23.06.2019 05:30
Suppose you discovered a new element with 120 protons and 2 electrons in its outer level . i'm what group does this new element belong? what properties would you expect it to have
Answers: 1
You know the right answer?
Carbon dioxide gas is collected at 27.0(degrees)c in an evacuated flask with a measured volume of 30...
Questions




Mathematics, 21.05.2021 08:40

Mathematics, 21.05.2021 08:40


Mathematics, 21.05.2021 08:40

English, 21.05.2021 08:40

Mathematics, 21.05.2021 08:40


Mathematics, 21.05.2021 08:40

Biology, 21.05.2021 08:40

Mathematics, 21.05.2021 08:40

Health, 21.05.2021 08:40

Mathematics, 21.05.2021 08:40


Mathematics, 21.05.2021 08:40