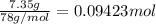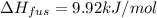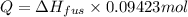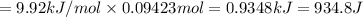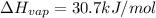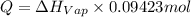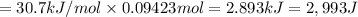Calculate the heat required to melt 7.35 g of benzene at its normal melting point. heat of fusion (benzene) = 9.92 kj/mol heat = kj
calculate the heat required to vaporize 7.35 g of benzene at its normal boiling point. heat of vaporization (benzene) = 30.7 kj/mol heat = kj

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2


Chemistry, 22.06.2019 15:30
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b.colder climates near the equator c.large waves on the cost of europe d.warm climates in northern europe
Answers: 1

Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3
You know the right answer?
Calculate the heat required to melt 7.35 g of benzene at its normal melting point. heat of fusion (b...
Questions

English, 01.04.2021 01:20

Mathematics, 01.04.2021 01:20

Mathematics, 01.04.2021 01:20

Biology, 01.04.2021 01:20




English, 01.04.2021 01:20

Social Studies, 01.04.2021 01:20



Mathematics, 01.04.2021 01:20

Business, 01.04.2021 01:20

Mathematics, 01.04.2021 01:20


Mathematics, 01.04.2021 01:20

Mathematics, 01.04.2021 01:20

Mathematics, 01.04.2021 01:20


Mathematics, 01.04.2021 01:20