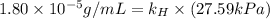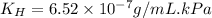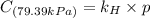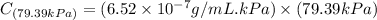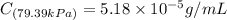Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
What is the maximum number of electrons that an atomic orbital can contain?
Answers: 1


Chemistry, 22.06.2019 09:20
Explain that newton first law,second law and third law of motion?
Answers: 2

Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2
You know the right answer?
Exposing a 250 ml sample of water at 20.∘c to an atmosphere containing a gaseous solute at 27.59 kpa...
Questions



Computers and Technology, 01.04.2020 20:30





Chemistry, 01.04.2020 20:30

Mathematics, 01.04.2020 20:30


History, 01.04.2020 20:30

Mathematics, 01.04.2020 20:30





History, 01.04.2020 20:30

Mathematics, 01.04.2020 20:30


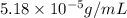
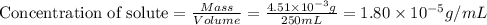
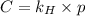
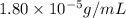
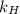 = Henry's law constant = ?
= Henry's law constant = ?