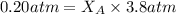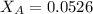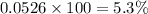Chemistry, 16.12.2019 17:31 kevinkingpin
Helium is mixed with oxygen gas for deep-sea divers. calculate the percent by volume of oxygen gas in the mixture if the diver has to submerge to a depth where the total pressure is 3.8 atm. the partial pressure of oxygen is maintained at 0.20 atm at this depth.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

Chemistry, 22.06.2019 20:30
Which of the following is not true about the atomic model of substances?
Answers: 1

Chemistry, 23.06.2019 03:00
Is it safe to take 450mg of diphenhydramine hydrochloride?
Answers: 1

Chemistry, 23.06.2019 03:10
Which of the following compounds would be expected to have the strongest ionic bonds? a)the compound that has b)the largest ions with the greatest charge c)the compound that has d)the largest ions with the least charge the compound that has the smallest ions with the greatest charge the compound that has the smallest ions with the least charge
Answers: 2
You know the right answer?
Helium is mixed with oxygen gas for deep-sea divers. calculate the percent by volume of oxygen gas i...
Questions

Chemistry, 19.04.2021 16:30


History, 19.04.2021 16:30





Mathematics, 19.04.2021 16:30

Mathematics, 19.04.2021 16:30

Mathematics, 19.04.2021 16:40

Mathematics, 19.04.2021 16:40

Computers and Technology, 19.04.2021 16:40


Mathematics, 19.04.2021 16:40

Mathematics, 19.04.2021 16:40

Social Studies, 19.04.2021 16:40

Mathematics, 19.04.2021 16:40

Mathematics, 19.04.2021 16:40


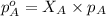
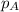 = total partial pressure of solution = 3.8 atm
= total partial pressure of solution = 3.8 atm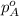 = partial pressure of oxygen = 0.20 atm
= partial pressure of oxygen = 0.20 atm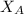 = mole fraction of oxygen = ?
= mole fraction of oxygen = ?