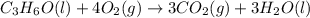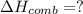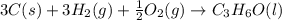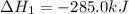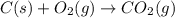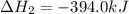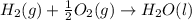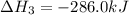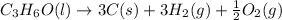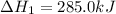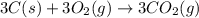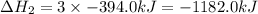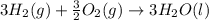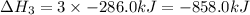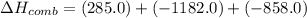Chemistry, 16.12.2019 17:31 gildedav001
What is the heat of reaction (δh°rxn) for the combustion of acetone (c3h6o) given the following thermochemical equations? 1. 3 c(s) + 3 h2(g) + ½ o2(g) → c3h6o(ℓ) δhf° = −285.0 kj 2. c(s) + o2(g) → co2(g) δhf° = −394.0 kj 3. h2(g) + ½ o2(g) → h2o(ℓ) δhf° = −286.0 kj

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Match each object to its description: a. coma of a comet b. comet tail c. oort cloud haze surrounding a nucleus created by solar wind. hypothetical sphere around the solar system
Answers: 1



You know the right answer?
What is the heat of reaction (δh°rxn) for the combustion of acetone (c3h6o) given the following ther...
Questions

Chemistry, 04.11.2020 07:20

Mathematics, 04.11.2020 07:20


Mathematics, 04.11.2020 07:20




Mathematics, 04.11.2020 07:20

Mathematics, 04.11.2020 07:20

History, 04.11.2020 07:20



Mathematics, 04.11.2020 07:20





Mathematics, 04.11.2020 07:20

Physics, 04.11.2020 07:20

 will be -1775 kJ
will be -1775 kJ