
Chemistry, 14.12.2019 07:31 andrejr0330jr
Nitrogen and oxygen can react to form various compounds. two experiments showed that one compound is formed when 3.62 g of nitrogen and 2.07 g of oxygen react completely, while another compound is formed when 1.82 g of nitrogen reacts completely with 4.13 g of oxygen. which of the following are most likely the molecular formulas for the nitrogen oxides obtained in these experiments? (1) no, n2o (2) no, no2 (3) n2o, n2o5 (4) no, n2o4 (5) n2o, n2o4

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3

Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1

Chemistry, 22.06.2019 22:00
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1
You know the right answer?
Nitrogen and oxygen can react to form various compounds. two experiments showed that one compound is...
Questions

Geography, 25.08.2021 19:20


Mathematics, 25.08.2021 19:20

Mathematics, 25.08.2021 19:20

Mathematics, 25.08.2021 19:20

Spanish, 25.08.2021 19:20



Mathematics, 25.08.2021 19:20


Mathematics, 25.08.2021 19:20

Mathematics, 25.08.2021 19:20


Biology, 25.08.2021 19:20

Mathematics, 25.08.2021 19:20

Physics, 25.08.2021 19:20

Mathematics, 25.08.2021 19:20

Mathematics, 25.08.2021 19:20


Computers and Technology, 25.08.2021 19:30

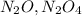
 .
.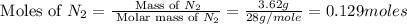
 .
.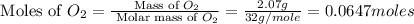
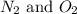 .
.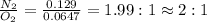
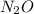 .
.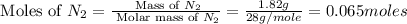
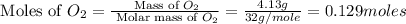
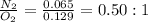
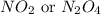 .
.

