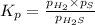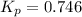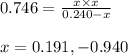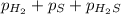Chemistry, 14.12.2019 06:31 nettaboo4664
At a certain temperature, the k p kp for the decomposition of h 2 s h2s is 0.746 0.746 . h 2 s ( g ) − ⇀ ↽ − h 2 ( g ) + s ( g ) h2s(g)↽−−⇀h2(g)+s(g) initially, only h 2 s h2s is present at a pressure of 0.240 0.240 bar in a closed container. what is the total pressure in the container at equilibrium?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 3

Chemistry, 23.06.2019 01:30
Use the periodic table to determine how many grams of oxygen would be required to react completely with 859.0 g c2h2
Answers: 3

Chemistry, 23.06.2019 03:00
In which of the following phases of matter do molecules have the highest amount of energy? a. liquid b. gel c. solid d. gas
Answers: 2

Chemistry, 23.06.2019 04:00
Calculate the mass of 0.750 mol of the following substance. na3po4. , i'm not quite sure on how to set up the problem to solve! : (
Answers: 1
You know the right answer?
At a certain temperature, the k p kp for the decomposition of h 2 s h2s is 0.746 0.746 . h 2 s ( g )...
Questions

Mathematics, 18.12.2019 03:31




Mathematics, 18.12.2019 03:31





Mathematics, 18.12.2019 03:31



History, 18.12.2019 03:31

Physics, 18.12.2019 03:31


History, 18.12.2019 03:31


Mathematics, 18.12.2019 03:31

History, 18.12.2019 03:31

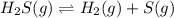
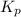 for above equation follows:
for above equation follows: