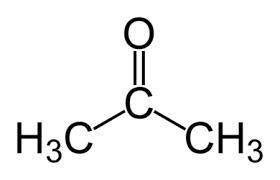
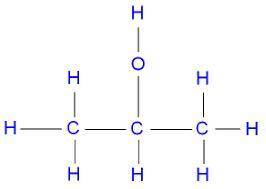
Identify the oxidation number of the highlighted carbon atoms in each of the molecules.
...

Chemistry, 14.12.2019 06:31 2023jpeterson
Identify the oxidation number of the highlighted carbon atoms in each of the molecules.
a. in molecule a a central carbon atom is bonded to two c h 3 groups and an o atom through a double bond.
b. the central carbon atom is highlighted.
c. in molecule b, a central carbon atom is bonded to two c h 3 groups, an o h group, and an h atom.
d. the central carbon atom is highlighted.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

You know the right answer?
Questions

Mathematics, 15.01.2020 14:31




Mathematics, 15.01.2020 14:31



Mathematics, 15.01.2020 14:31




Mathematics, 15.01.2020 14:31

Physics, 15.01.2020 14:31



History, 15.01.2020 14:31


Mathematics, 15.01.2020 14:31


Mathematics, 15.01.2020 14:31

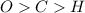
 always gets -1 and
always gets -1 and 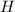 always gets +1 in these two compounds.The carbon - carbon bond will give 0 oxidation state.
always gets +1 in these two compounds.The carbon - carbon bond will give 0 oxidation state.