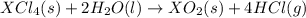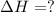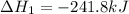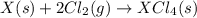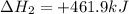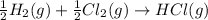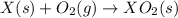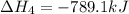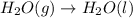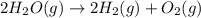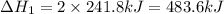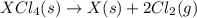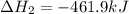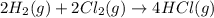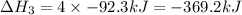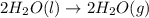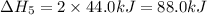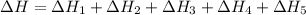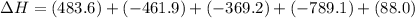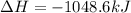Chemistry, 14.12.2019 05:31 ruddymorales1123
Given these reactions, where x represents a generic metal or metalloid 1 ) h 2 ( g ) + 1 2 o 2 ( g ) ⟶ h 2 o ( g ) δ h 1 = − 241.8 kj 2 ) x ( s ) + 2 cl 2 ( g ) ⟶ xcl 4 ( s ) δ h 2 = + 461.9 kj 3 ) 1 2 h 2 ( g ) + 1 2 cl 2 ( g ) ⟶ hcl ( g ) δ h 3 = − 92.3 kj 4 ) x ( s ) + o 2 ( g ) ⟶ xo 2 ( s ) δ h 4 = − 789.1 kj 5 ) h 2 o ( g ) ⟶ h 2 o ( l ) δ h 5 = − 44.0 kj what is the enthalpy, δ h , for this reaction? xcl 4 ( s ) + 2 h 2 o ( l ) ⟶ xo 2 ( s ) + 4 hcl ( g )

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
How much heat is released upon converting one mole of steam (18.0 g) from 100.0 ∘c to water at 25.0 ∘c? show work and constants, trying to figure out how it works. only given the heat capacity for steam and water so try to only use that
Answers: 1

Chemistry, 21.06.2019 22:30
In order to calculate the amount of heat transferred you must know the __ and specific heat of the material, as well as the change in temperature. a. volume b. density c. mass d. enthalpy
Answers: 1

Chemistry, 22.06.2019 02:00
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1
You know the right answer?
Given these reactions, where x represents a generic metal or metalloid 1 ) h 2 ( g ) + 1 2 o 2 ( g )...
Questions

Physics, 20.10.2020 16:01

Mathematics, 20.10.2020 16:01



Mathematics, 20.10.2020 16:01


Mathematics, 20.10.2020 16:01


Mathematics, 20.10.2020 16:01


Mathematics, 20.10.2020 16:01

Mathematics, 20.10.2020 16:01



English, 20.10.2020 16:01

Engineering, 20.10.2020 16:01

History, 20.10.2020 16:01

Computers and Technology, 20.10.2020 16:01

Biology, 20.10.2020 16:01

Chemistry, 20.10.2020 16:01