
Chemistry, 14.12.2019 01:31 orlando19882000
Calculate the ph of a 0.208 0.208 m solution of ethylenediamine ( h 2 nch 2 ch 2 nh 2 h2nch2ch2nh2). the p k a pka values for the acidic form of ethylenediamine ( h + 3 nch 2 ch 2 nh + 3 h3+nch2ch2nh3+) are 6.848 6.848 ( p k a1 pka1) and 9.928 9.928 ( p k a2 pka2). ph = ph= calculate the concentration of each form of ethylenediamine in this solution at equilibrium.
[ h 2 nch 2 ch 2 nh 2 ] =
[h2nch2ch2nh2]= m m
[ h 2 nch 2 ch 2 nh + 3 ] =
[h2nch2ch2nh3+]= m m
[ h + 3 nch 2 ch 2 nh + 3 ] =
[h3+nch2ch2nh3+]=

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1

Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1
You know the right answer?
Calculate the ph of a 0.208 0.208 m solution of ethylenediamine ( h 2 nch 2 ch 2 nh 2 h2nch2ch2nh2)....
Questions

Mathematics, 17.10.2020 14:01

Mathematics, 17.10.2020 14:01

Mathematics, 17.10.2020 14:01

Mathematics, 17.10.2020 14:01


Social Studies, 17.10.2020 14:01

Computers and Technology, 17.10.2020 14:01


Mathematics, 17.10.2020 14:01


History, 17.10.2020 14:01

Mathematics, 17.10.2020 14:01

English, 17.10.2020 14:01

History, 17.10.2020 14:01

Mathematics, 17.10.2020 14:01

Mathematics, 17.10.2020 14:01



Mathematics, 17.10.2020 14:01

Mathematics, 17.10.2020 14:01

![[H_{2}NCH_{2}NCH_{2}NH_{2}]](/tpl/images/0417/9676/3fc2a.png) = 0.204 M;
= 0.204 M;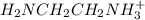 =
= 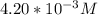
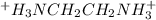 =
= 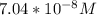
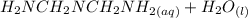 ⇔
⇔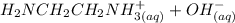
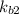 equation (1)
equation (1)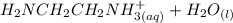 ⇔
⇔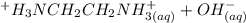
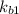 equation (2)
equation (2)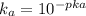
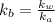

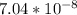
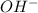 = x
= x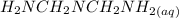 = 0.208-x
= 0.208-x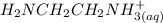 = x
= x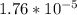
![[H_{2}NCH_{2}NCH_{2}NH_{2(aq)}]](/tpl/images/0417/9676/0b733.png) = 0.208 -
= 0.208 - 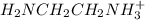
 = 2.38
= 2.38

