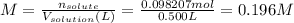Chemistry, 13.12.2019 18:31 Gigglygoose3159
You prepare a solution by dissolving 3.9280 g of solid sodium hydroxide in 300 ml of distilled water in a 500 ml volumetric flask. the flask is filled to the calibration line with distilled water, stoppered, inverted and swirled several times to thoroughly mix. the prepared solution is placed into a buret and the buret is clamped onto a ring stand. the initial volume of sodium hydroxide solution in the buret is 50 ml. calculate the concentration of the sodium hydroxide solution as prepared. be sure to include the appropriate unit abbreviation and leave a space between the number and the unit!

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 22.06.2019 08:30
The mass of a neutron is equal to the mass of a proton plus the mass of an electron. true or false false true
Answers: 1

Chemistry, 22.06.2019 11:30
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3
You know the right answer?
You prepare a solution by dissolving 3.9280 g of solid sodium hydroxide in 300 ml of distilled water...
Questions

Mathematics, 22.10.2020 21:01






History, 22.10.2020 21:01


Mathematics, 22.10.2020 21:01



History, 22.10.2020 21:01

Spanish, 22.10.2020 21:01

English, 22.10.2020 21:01

Mathematics, 22.10.2020 21:01


English, 22.10.2020 21:01



Mathematics, 22.10.2020 21:01