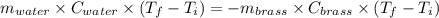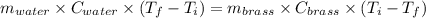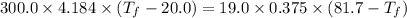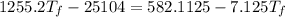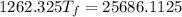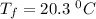Chemistry, 13.12.2019 06:31 BlueLemonWater
A19.0g sample of brass, which has a specific heat capacity of 0.375·j*g^−1°c−, is dropped into an insulated container containing 300.0g of water at 20.0°c and a constant pressure of 1atm. the initial temperature of the brass is 81.7°c.
1. assuming no heat is absorbed from or by the container, or the surroundings, calculate the equilibrium temperature of the water. be sure your answer has significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Select the correct answer. you have a nightlight plugged into an outlet in the hallway, which uses 3.5 watts when plugged in. if the house circuit provides 120.0 volts, what is the current through this bulb?
Answers: 1


Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2
You know the right answer?
A19.0g sample of brass, which has a specific heat capacity of 0.375·j*g^−1°c−, is dropped into an in...
Questions

History, 05.11.2020 20:10



English, 05.11.2020 20:10

Biology, 05.11.2020 20:10

Biology, 05.11.2020 20:10

Arts, 05.11.2020 20:10

Social Studies, 05.11.2020 20:10

Biology, 05.11.2020 20:10

Mathematics, 05.11.2020 20:10

Social Studies, 05.11.2020 20:10

Business, 05.11.2020 20:10

Medicine, 05.11.2020 20:10






Mathematics, 05.11.2020 20:10

History, 05.11.2020 20:10