Chemistry, 13.12.2019 06:31 antonjas001
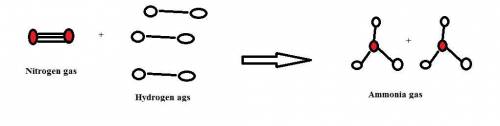
1. how many molecules of ammonia can be created when four molecules of nitrogen are combined with four molecules of hydrogen? include a drawing of the molecules in your answer.
2. what ratio of nitrogen and hydrogen molecules would result in no left-over reactants? explain your answer
3. if 100.0g of nitrogen is reacted with 100.0g of hydrogen, what is the theoretical yield of the reaction? what is the excess reactant? what is the limiting reactant?
me i'm so confused

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:00
For each of the following types of reactions, write a general reaction formula in the symbolic form—for example, a + b → ab. single-displacement double-displacement synthesis decomposition
Answers: 1

Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

You know the right answer?
1. how many molecules of ammonia can be created when four molecules of nitrogen are combined with fo...
Questions



Chemistry, 24.09.2019 09:30


Geography, 24.09.2019 09:30

Geography, 24.09.2019 09:30

Mathematics, 24.09.2019 09:30

Biology, 24.09.2019 09:30


Biology, 24.09.2019 09:30


Mathematics, 24.09.2019 09:30


Geography, 24.09.2019 09:30



Mathematics, 24.09.2019 09:30

Mathematics, 24.09.2019 09:30

Chemistry, 24.09.2019 09:30

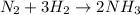
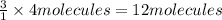 of hydrogen gas.
of hydrogen gas.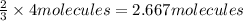 of ammonia
of ammonia = 1 : 3
= 1 : 3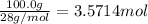
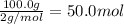
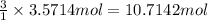 of hydrogen gas.
of hydrogen gas.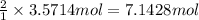 of ammonia
of ammonia