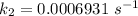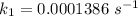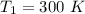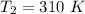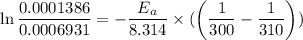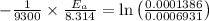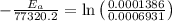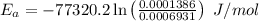Chemistry, 13.12.2019 06:31 supermimi8078
Areaction is known to exhibit 1st order kinetics. at 300k the concentration of reactant is reduced to one half of its initial value after 5000s. in contrast, at 310k the conc. is halved after 1000s. using this information to calculate: i) the rate constant for the reaction at 300kii) the time required for the reaction to be reduced to halfiii) the activation energy for the reaction

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1

Chemistry, 23.06.2019 00:30
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1

Chemistry, 23.06.2019 02:30
Which of the four hypothetical substances you investigated would be most harmful to living organisms? 50 points!
Answers: 2

Chemistry, 23.06.2019 03:30
The molar mass of nickel(ni) is 58.7 g/mol. how many moles are in an 88 gram sample of nickel?
Answers: 1
You know the right answer?
Areaction is known to exhibit 1st order kinetics. at 300k the concentration of reactant is reduced t...
Questions



Computers and Technology, 20.01.2022 18:00





Mathematics, 20.01.2022 18:00



Biology, 20.01.2022 18:00

Mathematics, 20.01.2022 18:00






History, 20.01.2022 18:10


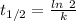
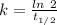
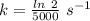
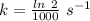
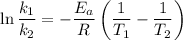

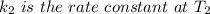
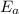 is the activation energy
is the activation energy