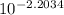Chemistry, 13.12.2019 03:31 smithsa10630
Electrochemical cells can be used to measure ionic concentrations. a cell is set up with a pair of zn electrodes, each immersed in znso4 solution. one solution is created to be exactly 0.500 m, and the potential on its electrode is measured to be 0.065 v higher than the other electrode. what is the concentration of the other solution? enter your answer in m.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 23.06.2019 06:00
Jenny wants to test the electrical conductivity of two substances dissolved in water. she is preparing the containers for the experiment. which factor is most important for her to control?
Answers: 1

Chemistry, 23.06.2019 06:30
The polarity of an oxygen-hydrogen bond is higher than the polarity of a nitrogen-hydrogen bond, allowing amines to be more soluble than alcohols.
Answers: 3

Chemistry, 23.06.2019 09:20
La reaccion entre monoxido de nitrogeno (no) y oxigeno para formardioxido de nitrogeno (no2) es un paso determinante para la formacion del smog, la reaccion es la siguiente: 2no + o2 = 2no2 cual sera el numero de moles de no2 que se formaran por la reaccion completa de 8 moles de oxigeno con suficiente monoxido?
Answers: 1
You know the right answer?
Electrochemical cells can be used to measure ionic concentrations. a cell is set up with a pair of z...
Questions

English, 06.01.2021 23:30

Mathematics, 06.01.2021 23:30


History, 06.01.2021 23:30

Mathematics, 06.01.2021 23:30

Mathematics, 06.01.2021 23:30


Mathematics, 06.01.2021 23:30


Chemistry, 06.01.2021 23:30



Mathematics, 06.01.2021 23:30

Health, 06.01.2021 23:30


Mathematics, 06.01.2021 23:30

English, 06.01.2021 23:30


Mathematics, 06.01.2021 23:30