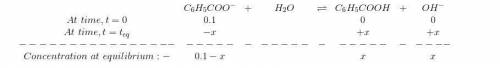Sodium benzoate (c6h5coona), the sodium salt of the weak acid benzoic acid, is used as a food preservative. a solution is prepared by dissolving 0.100 mol of sodium benzoate in enough pure water to produce 1.00 l of solution. if the pka for benzoic acid is 4.20, calculate the ph of the sodium benzoate solution.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2


Chemistry, 22.06.2019 21:30
While in europe, if you drive 125 km per day, how much money would you spend on gas in one week if gas costs 1.10 euros per liter and your car’s gas mileage is 32.0 mi/gal? assume that 1 euro=1.26 dollars
Answers: 2
You know the right answer?
Sodium benzoate (c6h5coona), the sodium salt of the weak acid benzoic acid, is used as a food preser...
Questions

History, 31.01.2020 11:00

Mathematics, 31.01.2020 11:00





Mathematics, 31.01.2020 11:00

English, 31.01.2020 11:00


Mathematics, 31.01.2020 11:00

Chemistry, 31.01.2020 11:00



Social Studies, 31.01.2020 11:00

English, 31.01.2020 11:00

Mathematics, 31.01.2020 11:00

Mathematics, 31.01.2020 11:00

Physics, 31.01.2020 11:00

Mathematics, 31.01.2020 11:00

Mathematics, 31.01.2020 11:00

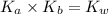
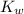 is the dissociation constant of water.
is the dissociation constant of water.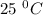 ,
, 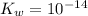
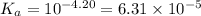
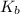 for Sodium benzoate can be calculated as:
for Sodium benzoate can be calculated as: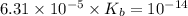
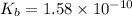
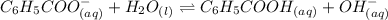
![K_{b}=\frac {\left [ C_6H_5COOH^{+} \right ]\left [ {OH}^- \right ]}{[C_6H_5COO^-]}](/tpl/images/0416/3772/af673.png)
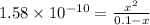
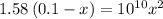
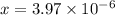
![[OH^-]=3.97\times 10^{-6}](/tpl/images/0416/3772/71435.png)
![pOH=-log[OH^-]=-log(3.97\times 10^{-6})=5.4](/tpl/images/0416/3772/9fc03.png)