
Chemistry, 13.12.2019 01:31 terrysizemore666
The melting points for the second series transition elements increase from 1526˚c for yttrium to 2623˚c for molybdenum and then decrease again to 321˚c for cadmium. account for this trend in terms of band theory.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
What pressure will be exerted by 0.675 moles of a gas at 25*c if it is in a 0.750-l container?
Answers: 1

Chemistry, 22.06.2019 01:30
100 points answer quick the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1


Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1
You know the right answer?
The melting points for the second series transition elements increase from 1526˚c for yttrium to 262...
Questions



Mathematics, 11.03.2020 18:41






English, 11.03.2020 18:41


Computers and Technology, 11.03.2020 18:41


Social Studies, 11.03.2020 18:41

Social Studies, 11.03.2020 18:41






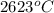 and after that the electrons goes into the antibonding and reaches minimum for cadmium when the anti boning orbital is completely filled. So, the melting point of the cadmium is tex]321^{o}C[/tex].
and after that the electrons goes into the antibonding and reaches minimum for cadmium when the anti boning orbital is completely filled. So, the melting point of the cadmium is tex]321^{o}C[/tex].

