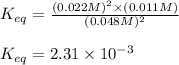Chemistry, 13.12.2019 00:31 JohnJamesPaksitani
Hydrogen peroxide may decompose to form water and oxygen gas according to the following reaction 2h202(g) 2h20(g)+02(g) in a particular experiment, 1.75 moles of hy02 were placed in a 25-l reaction chamber at 307ec after equilibrium was reached, 1.20 moles of h202 remained what is ke for the reaction?
a. 2.4×10^-3
b. 2.0×10^-4
c. 5.5×10^-3
d. 2.3×10^-2

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 23.06.2019 00:30
Nuclear decay is the spontaneous decay of one element into a. an x-ray b. a ray of light c. another element
Answers: 1

Chemistry, 23.06.2019 12:00
Explaining why atoms bondcomplete the sentence.atoms form chemical bonds to satisfy the rule and to become .
Answers: 1
You know the right answer?
Hydrogen peroxide may decompose to form water and oxygen gas according to the following reaction 2h2...
Questions

Social Studies, 14.12.2020 22:10

Mathematics, 14.12.2020 22:10

Biology, 14.12.2020 22:10

History, 14.12.2020 22:10

Social Studies, 14.12.2020 22:10




Chemistry, 14.12.2020 22:10


Biology, 14.12.2020 22:10


Arts, 14.12.2020 22:10







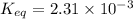
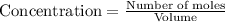
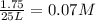
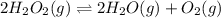
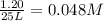
![[H_2O]=2x=2\times 0.011 M= 0.022 M](/tpl/images/0416/0677/428c7.png)
![[O_2]=x=0.011 M](/tpl/images/0416/0677/5d441.png)
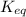 for the above reaction follows:
for the above reaction follows:![K_{eq}=\frac{[H_2O]^2\times [O_2]}{ [H_2O_2]^2}](/tpl/images/0416/0677/4f6fe.png)