Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1

Chemistry, 22.06.2019 19:50
What is the wavelength of a wave with a velocity of 50 m/s and a frequency of 5hz a 250 m b 0.1 m c 10m d 0.01 m
Answers: 2
You know the right answer?
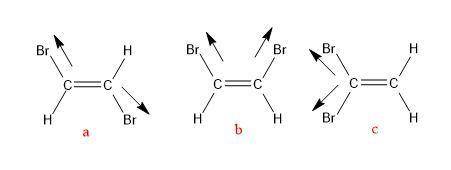
There are three different possible isomers of a dibromoethene molecule, c2h2br2c2h2br2 . one of them...
Questions


Chemistry, 17.12.2021 02:40

Chemistry, 17.12.2021 02:40

Mathematics, 17.12.2021 02:40



Mathematics, 17.12.2021 02:40


Mathematics, 17.12.2021 02:40


Mathematics, 17.12.2021 02:40

Mathematics, 17.12.2021 02:40

Mathematics, 17.12.2021 02:40


Mathematics, 17.12.2021 02:40

SAT, 17.12.2021 02:40


Mathematics, 17.12.2021 02:40