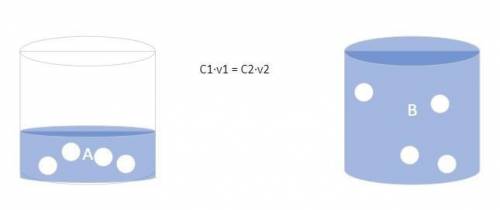
The forensic technician at a crime scene has just prepared a luminol stock solution by adding 18.0 g of luminol to h2o creating a solution with a total volume of 75.0 ml. what is the molarity of the stock solution of luminol? before investigating the scene, the technician must dilute the luminol solution to a concentration of 5.00×10^-2 m. the diluted solution is then placed in a spray bottle for application on the desired surfaces. how many moles of luminol are present in 2.00 l of the diluted spray? what volume of the stock solution (part a) would contain the number of moles present in the diluted solution (part b)?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 12:00
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1
You know the right answer?
The forensic technician at a crime scene has just prepared a luminol stock solution by adding 18.0 g...
Questions


History, 11.06.2021 04:20





English, 11.06.2021 04:20


Mathematics, 11.06.2021 04:20

Mathematics, 11.06.2021 04:20


Social Studies, 11.06.2021 04:20


History, 11.06.2021 04:20

Mathematics, 11.06.2021 04:20

English, 11.06.2021 04:20

Mathematics, 11.06.2021 04:20