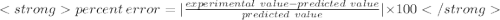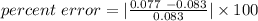Chemistry, 12.12.2019 21:31 batmandillon21
Silver was precipitated and collected by filtration. the experiment yielded .077g of silver after dying. the predicted yield was .083g. the percent error present in the collection would be ?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Will give brainliest it is a lab from k12 here is the linkfor each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. type your answer here. (score for question 3: of 5 points) were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. type your answer here. (score for question 4: of 5 points) make a general statement about the reactivity of the metals in this experiment. type your answer here.
Answers: 2


Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 22.06.2019 22:30
Draw the aromatic compound toluene (methylbenzene). show all hydrogen atoms, including those on the ring.
Answers: 1
You know the right answer?
Silver was precipitated and collected by filtration. the experiment yielded .077g of silver after dy...
Questions




Computers and Technology, 21.11.2020 03:20

Arts, 21.11.2020 03:20

History, 21.11.2020 03:20

Biology, 21.11.2020 03:20

Social Studies, 21.11.2020 03:20


Mathematics, 21.11.2020 03:20

Mathematics, 21.11.2020 03:20


History, 21.11.2020 03:20

Social Studies, 21.11.2020 03:30


Mathematics, 21.11.2020 03:30

English, 21.11.2020 03:30