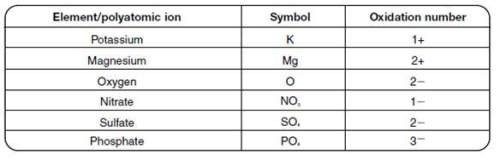
Use the table to determine what the correct formula for magnesium oxide is.
...

Chemistry, 12.12.2019 20:31 dieulynx1171
Use the table to determine what the correct formula for magnesium oxide is.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:10
There are 6.022 x 10^23 atoms of hg in 1 mole of hg. the number of atoms in 4.5 moles of hg can be found by multiplying 4.5 by 6.022 x 10^23 a. 2.7 x 10^24 b. 27 x 10^23 c. 2.71 x10^24 d. 27.099 x 10^23
Answers: 3

Chemistry, 21.06.2019 19:30
If the element whose electric configuration ends in the d sublevel, the element is calssified as? a.inner transition b.noble gases c.representative d. transition
Answers: 2


Chemistry, 21.06.2019 22:30
Each of the following compounds contains a metal that can exhibit more than one ionic charge. provide systematic names for each of these compounds. (a) cr(clo3)6 (b) mo(cn)6 (c) cr2(so3)3 (d) v(clo2)2 (e) v(cn)5 (f) os(clo2)4
Answers: 3
You know the right answer?
Questions



Mathematics, 16.12.2020 21:30



Mathematics, 16.12.2020 21:30


Mathematics, 16.12.2020 21:30


History, 16.12.2020 21:30

Mathematics, 16.12.2020 21:30

Mathematics, 16.12.2020 21:30


Chemistry, 16.12.2020 21:30

Mathematics, 16.12.2020 21:30





Mathematics, 16.12.2020 21:30



