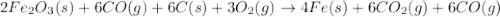Blast furnaces extract pure iron from the iron(iii) oxide in iron ore in a two step sequence. in the first step, carbon and oxygen react to form carbon monoxide: 2c (s) + o2 (g) → 2co (g)in the second step, iron(iii) oxide and carbon monoxide react to form iron and carbon dioxide: fe2o3 (s) + 3co (g) → 2fe (s) + 3co2 (g)write the net chemical equation for the production of iron from carbon, oxygen and iron(iii) oxide. be sure your equation is balanced.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

You know the right answer?
Blast furnaces extract pure iron from the iron(iii) oxide in iron ore in a two step sequence. in the...
Questions




English, 02.10.2019 08:30

English, 02.10.2019 08:30



History, 02.10.2019 08:30

Mathematics, 02.10.2019 08:30

History, 02.10.2019 08:30


Mathematics, 02.10.2019 08:30

English, 02.10.2019 08:30


Spanish, 02.10.2019 08:30





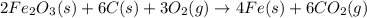
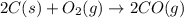 ..[1]
..[1]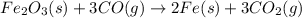 ..[2]
..[2]