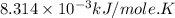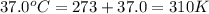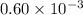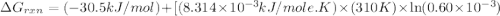Chemistry, 12.12.2019 05:31 eileentennyson
Acritical reaction in the production of energy to do work or drive chemical reactions in biological systems is the hydrolysis of adenosine triphosphate, atp, to adenosine diphosphate, adp, as described by the reaction atp ( aq ) + h 2 o ( l ) ⟶ adp ( aq ) + hpo 2 − 4 ( aq ) for which δ g ∘ rxn = − 30.5 kj/mol at 37.0 °c and ph 7.0. calculate the value of δ g rxn in a biological cell in which [ atp ] = 5.0 mm, [ adp ] = 0.60 mm, and [ hpo 2 − 4 ] = 5.0 mm.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:20
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l.s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3

Chemistry, 22.06.2019 19:40
What causes different colors to appear in the sky? the absorption of light by air molecules the reflection of light by bodies of water the greenhouse effect in earth's atmosphere the scattering and reflection of light by dust particles
Answers: 2

Chemistry, 23.06.2019 02:00
An alpha particle is: a hydrogen atom a nucleus of helium two neutrons an electron
Answers: 1

Chemistry, 23.06.2019 04:00
How much energy is required to vaporize 2 kg of copper? a 4730 kj b 207kj c 9460 kj d 414kj
Answers: 1
You know the right answer?
Acritical reaction in the production of energy to do work or drive chemical reactions in biological...
Questions


Spanish, 26.09.2019 23:30

Mathematics, 26.09.2019 23:30

Mathematics, 26.09.2019 23:30


Mathematics, 26.09.2019 23:30

English, 26.09.2019 23:30

Business, 26.09.2019 23:30


Social Studies, 26.09.2019 23:30


Mathematics, 26.09.2019 23:30


English, 26.09.2019 23:30


Physics, 26.09.2019 23:30


Biology, 26.09.2019 23:30


Chemistry, 26.09.2019 23:30

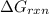 is -49.6 kJ/mol
is -49.6 kJ/mol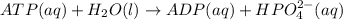
![Q=\frac{[ADP][HPO_4^{2-}]}{[ATP]}](/tpl/images/0414/8690/ccdf0.png)
![[ATP]](/tpl/images/0414/8690/bda18.png) = 5.0 mM
= 5.0 mM![[ADP]](/tpl/images/0414/8690/68360.png) = 0.60 mM
= 0.60 mM![[HPO_4^{2-}]](/tpl/images/0414/8690/c0ca9.png) = 5.0 mM
= 5.0 mM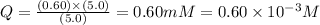
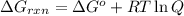 ............(1)
............(1)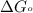 = standard Gibbs free energy = -30.5 kJ/mol
= standard Gibbs free energy = -30.5 kJ/mol