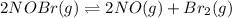Chemistry, 12.12.2019 04:31 dhurtado1195
For the following reaction, the equilibrium constant kc is 2.0 at a certain temperature. the reaction is endothermic. what do you expect to happen to the concentration of no if the temperature is doubled? 2nobr( g) ⇌ 2no( g) + br 2( g) the change in concentration of [no] will depend on the size of the vessel. the concentration of no will increase. the concentration of no will decrease. a catalyst will be needed to make a change in concentration. there will be no change in [no].

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which of these would be caused by a chemical change? a) the formation of lava. b) sedimantary rock layering over time. c) metamorphic rock forming from igneous. d) metamorphic rock eroding to form sedimentary rock.
Answers: 3

Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1

Chemistry, 23.06.2019 03:40
The following questions a24 - a26 relate to 100 ml of 0.0150 m solution of benzoic acid (c6h3cooh). ka(c6h3cooh) = 6.4 x 10^-5. what is the ph of the solution after the addition of 1 x 10^-3 moles of naoh? you may assume no volume change to the solution upon addition of the naoh.
Answers: 2

Chemistry, 23.06.2019 03:50
How many moles of potassium are needed to react completely with 12.8 moles of magnessium bromide?
Answers: 2
You know the right answer?
For the following reaction, the equilibrium constant kc is 2.0 at a certain temperature. the reactio...
Questions

Mathematics, 14.02.2021 01:00




Physics, 14.02.2021 01:00

Mathematics, 14.02.2021 01:00






Mathematics, 14.02.2021 01:00


Mathematics, 14.02.2021 01:00


Mathematics, 14.02.2021 01:00

Mathematics, 14.02.2021 01:00

Physics, 14.02.2021 01:00