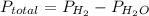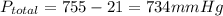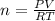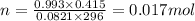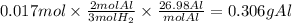Chemistry, 12.12.2019 04:31 paigebmaxwell6062
Solid aluminum reacts with aqueous h2so4 to form h2 gas and aluminum sulfate. when a sample of al is allowed to react, 415ml of gas is collected over water at 23°c at a pressure of 755mmhg. at 23°c the vapor pressure of water is 21mmhg. what is the pressure in mmhg of the dry h2 gas?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1

Chemistry, 23.06.2019 00:30
Which radioisotope is used to date fossils? a. oxygen-16 b. carbon-14 c. uranium-238 d. carbon-12
Answers: 2
You know the right answer?
Solid aluminum reacts with aqueous h2so4 to form h2 gas and aluminum sulfate. when a sample of al is...
Questions



Mathematics, 01.12.2019 07:31

SAT, 01.12.2019 07:31

English, 01.12.2019 07:31


Mathematics, 01.12.2019 07:31

Mathematics, 01.12.2019 07:31

English, 01.12.2019 07:31





Mathematics, 01.12.2019 07:31


Mathematics, 01.12.2019 07:31

Chemistry, 01.12.2019 07:31