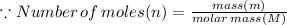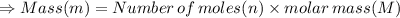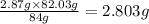Chemistry, 12.12.2019 03:31 jaylynomalley
In the stoichiometry laboratory experiment you reacted sodium bicarbonate with acetic acid to form sodium acetate, water and carbon dioxide according to the following equation. nahco3 + ch3cooh → h2o + co2 + nach3co2 calculate the theoretical yield of the reaction if you reacted 2.87 grams of sodium bicarbonate with sufficient acetic acid to produce sodium acetate. group of answer choices

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3


Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1
You know the right answer?
In the stoichiometry laboratory experiment you reacted sodium bicarbonate with acetic acid to form s...
Questions

English, 25.06.2019 17:30

Physics, 25.06.2019 17:30

Mathematics, 25.06.2019 17:30

Health, 25.06.2019 17:30


English, 25.06.2019 17:30






Biology, 25.06.2019 17:30

History, 25.06.2019 17:30


Mathematics, 25.06.2019 17:30

History, 25.06.2019 17:30

Mathematics, 25.06.2019 17:30