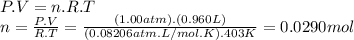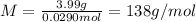Problem page a sample of an unknown compound is vaporized at . the gas produced has a volume of at a pressure of , and it weighs . assuming the gas behaves as an ideal gas under these conditions, calculate the molar mass of the compound. round your answer to significant digits. clears your work. undoes your last action. provides information about entering answers.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Aphysical reaction is a process in which one or more reactants change into one or more products with different properties. select the best answer from the choices provided t f
Answers: 1

Chemistry, 21.06.2019 22:00
To save time, you can approximate the initial mass of the solid to the nearest ±1 g. for example, if you are asked to add 14.3 g of copper, add between 13 g and 15 g. which of the following sets include two samples with an equal density? which all that apply below 15.4 g gold and 18.7 g silver 15.2 g copper and 50.0 g copper 20.2 g silver and 20.2 g copper 11.2 g gold and 14.9 g gold
Answers: 1

Chemistry, 21.06.2019 23:00
50 pts plz what is the physical state of matter of baking soda.
Answers: 1

Chemistry, 22.06.2019 05:30
Which other elements contain the same number of outer electrons as sodium
Answers: 3
You know the right answer?
Problem page a sample of an unknown compound is vaporized at . the gas produced has a volume of at a...
Questions

Mathematics, 03.12.2020 21:10

English, 03.12.2020 21:10





Biology, 03.12.2020 21:10


Mathematics, 03.12.2020 21:10

Mathematics, 03.12.2020 21:10

Mathematics, 03.12.2020 21:10


Mathematics, 03.12.2020 21:10


English, 03.12.2020 21:10





Social Studies, 03.12.2020 21:10