
An equilibrium mixture of pcl 5 ( g ) , pcl 3 ( g ) , and cl 2 ( g ) has partial pressures of 217.0 torr, 13.2 torr, and 13.2 torr, respectively. a quantity of cl 2 ( g ) is injected into the mixture, and the total pressure jumps to 263.0 torr. the appropriate chemical equation is pcl 3 ( g ) + cl 2 ( g ) − ⇀ ↽ − pcl 5 ( g ) calculate the new partial pressures after equilibrium is reestablished.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:10
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 20:30
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1

Chemistry, 22.06.2019 22:30
What is the value of the standard enthalpy of formation of an element in its most stable form?
Answers: 3
You know the right answer?
An equilibrium mixture of pcl 5 ( g ) , pcl 3 ( g ) , and cl 2 ( g ) has partial pressures of 217.0...
Questions


History, 14.07.2019 00:30


Mathematics, 14.07.2019 00:30

Mathematics, 14.07.2019 00:30

English, 14.07.2019 00:30

Chemistry, 14.07.2019 00:30

English, 14.07.2019 00:30




Mathematics, 14.07.2019 00:30

English, 14.07.2019 00:30


Mathematics, 14.07.2019 00:30



Biology, 14.07.2019 00:30

Mathematics, 14.07.2019 00:30

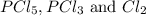 when equilibrium is re-established are 223.4 torr, 6.82 torr and 26.4 torr respectively.
when equilibrium is re-established are 223.4 torr, 6.82 torr and 26.4 torr respectively.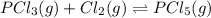
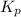 for above reaction follows:
for above reaction follows: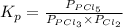 ........(1)
........(1)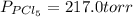
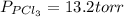
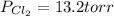
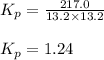
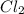 .
.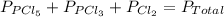
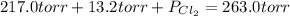
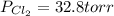
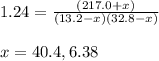
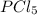 = (217.0+x) = (217.0+6.38) = 223.4 torr
= (217.0+x) = (217.0+6.38) = 223.4 torr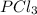 = (13.2-x) = (13.2-6.38) = 6.82 torr
= (13.2-x) = (13.2-6.38) = 6.82 torr


