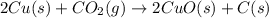The reaction 2cu(s) + co2(g) → 2cuo(s) + c(s) is endothermic. which of the following statements is true?
a) the reaction is not spontaneous at any temperature.
b) the reaction is spontaneous at all temperatures.
c) it is impossible to determine if the reaction is spontaneous without calculations.
d) the reaction will be spontaneous only at low temperatures.
e) the reaction will be spontaneous only at high temperatures.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3

Chemistry, 23.06.2019 10:00
Which number should be placed before f2 on the reactants side equation to make equation balanced? xe + > xef4
Answers: 1

Chemistry, 23.06.2019 12:30
Is the genie in the bottle experiment a physical or chemical change/reaction?
Answers: 1

Chemistry, 23.06.2019 12:50
What is the daughter nucleus produced a. when 217(at) undergoes alpha decay? b. when 103(mo) undergoes beta decay? c. when 188(hg) undergoes positron emission?
Answers: 1
You know the right answer?
The reaction 2cu(s) + co2(g) → 2cuo(s) + c(s) is endothermic. which of the following statements is t...
Questions

Mathematics, 17.10.2019 19:20





History, 17.10.2019 19:20


Social Studies, 17.10.2019 19:20

English, 17.10.2019 19:20

Mathematics, 17.10.2019 19:20

Mathematics, 17.10.2019 19:20



History, 17.10.2019 19:20


Geography, 17.10.2019 19:20


Mathematics, 17.10.2019 19:20

Arts, 17.10.2019 19:20