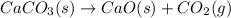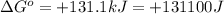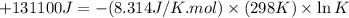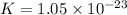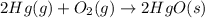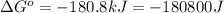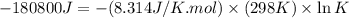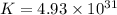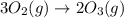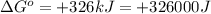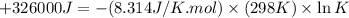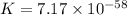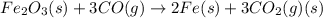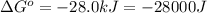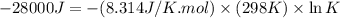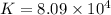Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 10:00
Why is the structure of molecule important to its function?
Answers: 1

Chemistry, 23.06.2019 06:00
Is the flow of energy during vaporizing more like the flow during melting or during freezing
Answers: 1

Chemistry, 23.06.2019 09:20
Due tomorrow which would have a lower ph, a 0.1 m solution of a strong base or a weak base? why? which would have a higher ph, a 0.1 m solution of a strong base or a weak base? why?
Answers: 3
You know the right answer?
Which of the following reactions will have the largest value of k at 298 k? a) caco3(s) → cao(s) +...
Questions

World Languages, 01.08.2021 23:10





Mathematics, 01.08.2021 23:10

Mathematics, 01.08.2021 23:10

Geography, 01.08.2021 23:10

Physics, 01.08.2021 23:10


Mathematics, 01.08.2021 23:10

Mathematics, 01.08.2021 23:10


Biology, 01.08.2021 23:10

World Languages, 01.08.2021 23:10





Mathematics, 01.08.2021 23:10

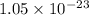
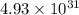
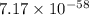
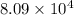
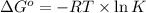
 = standard Gibbs free energy
= standard Gibbs free energy