
Chemistry, 11.12.2019 21:31 keniaguevara32
Calculate δhrxn for the following reaction: ch4(g)+2cl2(g)→ch2cl2(g)+2hcl(g) use the following reactions and given δh values. ch4(g)+cl2(g)→ch3cl(g)+hcl(g), δh=−99.60 kj ch3cl(g)+cl2(g)→ch2cl2(g)+hcl(g), δh=−105.8 kj express your answer to four significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 15:30
Plz me ! 1 which of earths spheres contains most of its mass? a atmosphere b hydrosphere c geosphere* d biosphere 2 erosion and weathering are examples of which types of forces? a constructive forces b destructive forces* c gravitational forces d inertia-related forces 3 which of the following statements about earths atmosphere is true? a earths atmosphere contains 78% water vapor which is essentail to life b earths atmosphere contains 21% oxygen c earths atmosphere contains carbon dioxide which all life forms require d earths atmosphere allows radiation from the sun to pass through it and warm earths surface* 4 the strenght of the force of gravity between two objects is determined by which of the following factors? select all that apply a the messes of the objects* b the distance between the objects* c the volumes of the objects d the surface area of the objects 5 earth and moon are kept in there respective orbits due to the influence of a inertia b gravity c gravity and inertia* d neither gravity or inertia if you answer all questions right i will give
Answers: 1


Chemistry, 23.06.2019 00:40
To prevent the presence of air, noble gases are placed over highly reactive chemicals to act as inert "blanketing" gases. a chemical engineer places a mixture of noble gases consisting of 4.37 g of he, 13.36 g of ne, and 36.65 g of kr in a piston-cylinder assembly at stp. calculate the partial pressure in torr of kr.
Answers: 1
You know the right answer?
Calculate δhrxn for the following reaction: ch4(g)+2cl2(g)→ch2cl2(g)+2hcl(g) use the following reac...
Questions



Physics, 04.10.2021 04:30


Chemistry, 04.10.2021 04:30

Mathematics, 04.10.2021 04:30

Mathematics, 04.10.2021 04:30

English, 04.10.2021 04:30


Physics, 04.10.2021 04:30


Mathematics, 04.10.2021 04:30



Physics, 04.10.2021 04:30


Mathematics, 04.10.2021 04:30


History, 04.10.2021 04:30

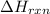 for the reaction is -205.4 kJ.
for the reaction is -205.4 kJ.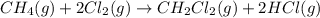
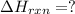
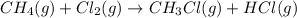
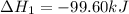
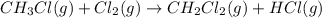
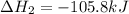
![\Delta H_{rxn}=[1\times \Delta H_1]+[1\times \Delta H_2]](/tpl/images/0414/0614/6e774.png)
![\Delta H_{rxn}=[(1\times (-99.60))+(1\times (-105.8))]=-205.4kJ](/tpl/images/0414/0614/3a575.png)


