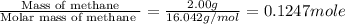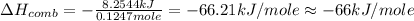Chemistry, 11.12.2019 19:31 wcraig1998
When 2.00 g of methane are burned in a bomb calorimeter, the change in temperature is 3.08°c. the heat capacity of the calorimeter is 2.68 kj/°c. the molar mass of methane is 16.042 g/mol. what is the approximate molar enthalpy of combustion of this substance?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2
You know the right answer?
When 2.00 g of methane are burned in a bomb calorimeter, the change in temperature is 3.08°c. the he...
Questions

Biology, 26.11.2019 14:31

Social Studies, 26.11.2019 14:31



Mathematics, 26.11.2019 14:31





English, 26.11.2019 14:31


Biology, 26.11.2019 14:31

Mathematics, 26.11.2019 14:31

History, 26.11.2019 14:31

Biology, 26.11.2019 14:31


Mathematics, 26.11.2019 14:31



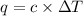
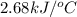
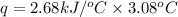
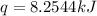
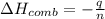
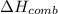 = enthalpy change = ?
= enthalpy change = ?