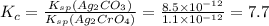Chemistry, 11.12.2019 19:31 mariahrpoulin1511
The value of the solubility product constant for ag2co3 is 8.5 × 10‒12 and that of ag2cro4 is 1.1 × 10‒12. from this data, what is the value of kc for the reaction, ag2co3(s) + cro42‒(aq) → ag2cro4(s) + co32‒(aq) a) 9.6 × 10‒12 b) 7.7 c) 1.1 × 1023 d) 1.3 × 10‒1 e) 9.4 × 10‒24

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3

Chemistry, 22.06.2019 23:30
Imagine a small synthetic vesicle made from pure phospholipids enclosing an interior lumen containing 1 mm glucose and 1 mm sodium chloride. if the vesicle is placed in pure water, which of the following happens faster? a. na+ diffuses out. b. cl– diffuses out. c. h2o diffuses in. d. glucose diffuses out. e. sodium chloride diffuses out.
Answers: 3


Chemistry, 23.06.2019 04:00
How much energy is required to vaporize 2 kg of copper? a 4730 kj b 207kj c 9460 kj d 414kj
Answers: 1
You know the right answer?
The value of the solubility product constant for ag2co3 is 8.5 × 10‒12 and that of ag2cro4 is 1.1 ×...
Questions






Mathematics, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30



Mathematics, 18.03.2021 01:30


Biology, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30

History, 18.03.2021 01:30


Mathematics, 18.03.2021 01:30

English, 18.03.2021 01:30

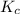 for the given reaction is 7.7
for the given reaction is 7.7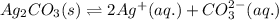
![K_{sp}(Ag_{2}CO_{3})=[Ag^{+}]^{2}[CO_{3}^{2-}]](/tpl/images/0413/8617/b8832.png)
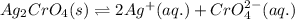
![K_{sp}(Ag_{2}CrO_{4})=[Ag^{+}]^{2}[CrO_{4}^{2-}]](/tpl/images/0413/8617/31f53.png)
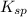 represents solubility product
represents solubility product![K_{c}=\frac{[CO_{3}^{2-}]}{[CrO_{4}^{2-}]}](/tpl/images/0413/8617/fea9e.png) (concentration of pure solids remain constant during reaction. Hence their concentration is taken as 1 to exclude them from equilibrium constant expression)
(concentration of pure solids remain constant during reaction. Hence their concentration is taken as 1 to exclude them from equilibrium constant expression)![K_{c}=\frac{[Ag^{+}]^{2}[CO_{3}^{2-}]}{[Ag^{+}]^{2}[CrO_{4}^{2-}]}](/tpl/images/0413/8617/3fc1b.png)