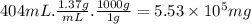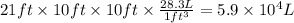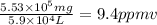Chemistry, 11.12.2019 18:31 serenityarts123
Aclumsy chemist drops a beaker containing 404 ml of a solvent (mw = 88.2, sg = 1.37) in a room measuring 21 ft x 10 ft x 10 ft. assuming that the contents of the beaker completely evaporate and fill the space, what is the resulting concentration in parts per million by volume (ppmv)? assume normal temperature and pressure (ntp), i. e., p = 1 atm, and t = 25 celsius. 1 ft3 = 28.3 l

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 12:50
Use the standard enthalpies of formation for the reactants and products to solve for the δhrxn for the following reaction. (the δhf of c2h4 is 52.26 kj/mol, co2 is -393.509 kj/mol, and h2o is -241.818 kj.) c2h4 (g) + 3o2(g) 2co2 (g) + 2h2o(g) δhrxn = the reaction is .
Answers: 3


Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 3
You know the right answer?
Aclumsy chemist drops a beaker containing 404 ml of a solvent (mw = 88.2, sg = 1.37) in a room measu...
Questions


Chemistry, 11.11.2020 20:30

Mathematics, 11.11.2020 20:30

Mathematics, 11.11.2020 20:30





Chemistry, 11.11.2020 20:30





Chemistry, 11.11.2020 20:30

Mathematics, 11.11.2020 20:30


Biology, 11.11.2020 20:30

Mathematics, 11.11.2020 20:30


Mathematics, 11.11.2020 20:30