
Acertain dose of tums contains 750.0 mg of calcium carbonate, caco3, in each tablet. in this problem, you will follow the steps to calculate how many grams of stomach acid, hcl, are neutralized by one tablet of tums. balance the chemical equation for the reaction of hcl with caco3. chemical equation: hcl + caco_{3} -> cacl_{2} + co_{2} + h_{2}o hcl+caco3⟶cacl2+co2+h2o
using the balanced equation, determine how many moles of hclhcl will react with 1 mol1 mol of caco3.caco3.
moles of hcl: hcl:
calculate how many moles 750.0 mg750.0 mg caco3caco3 is.
750.0 mg caco3=750.0 mg caco3= caco3
using your answers from the first two questions, calculate how many moles of hclhcl are neutralized when 750.0 mg750.0 mg caco3caco3 reacts.
moles of hcl: hcl:
convert the number of moles of hclhcl that you found in the previous question to grams of hcl. hcl. your answer is the amount (in grams) of hclhcl neutralized by one tablet of tums.
mass of hclhcl:

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 23:00
What is a substance? a. a physical property of matter b. a chemical property of matter c. an element or compound that cannot be physically separated d. characteristics used to tell the difference between mixtures
Answers: 1

Chemistry, 22.06.2019 23:30
Rank substituents in order of their priority when assigning the e or z label to an alkene. i, ch2i , h, ch2ch2cl, f
Answers: 2

Chemistry, 23.06.2019 03:30
Which of the following describes the entropy change as a solution is made from a liquid and solid
Answers: 1
You know the right answer?
Acertain dose of tums contains 750.0 mg of calcium carbonate, caco3, in each tablet. in this problem...
Questions

Social Studies, 23.08.2019 20:30

English, 23.08.2019 20:30

Spanish, 23.08.2019 20:30




English, 23.08.2019 20:30


Mathematics, 23.08.2019 20:30

Mathematics, 23.08.2019 20:30

Social Studies, 23.08.2019 20:30


Chemistry, 23.08.2019 20:30







Mathematics, 23.08.2019 20:30

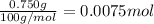
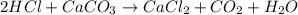
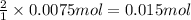 of HCl.
of HCl.

