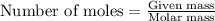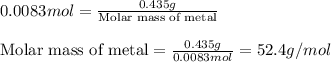A0.435 g sample of a metal, m, reacts completely with sulfuric acid according to m ( s ) + h 2 so 4 ( aq ) ⟶ mso 4 ( aq ) + h 2 ( g ) a volume of 201 ml of hydrogen is collected over water; the water level in the collecting vessel is the same as the outside level. atmospheric pressure is 756.0 torr, and the temperature is 25 °c. calculate the molar mass of the metal.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1


Chemistry, 23.06.2019 03:00
What volume does 1.70 ×10–3 mol of chlorine gas occupy if its temperature is 20.2 °c and its pressure is 795 mm hg?
Answers: 3

Chemistry, 23.06.2019 05:30
According to thomson, the atom is a positively charged cloud with electrons scattered throughout. what would the alpha particles do when they hit the foil if thomson were correct
Answers: 1
You know the right answer?
A0.435 g sample of a metal, m, reacts completely with sulfuric acid according to m ( s ) + h 2 so 4...
Questions

Mathematics, 28.12.2020 20:40

Computers and Technology, 28.12.2020 20:40

Engineering, 28.12.2020 20:40

Physics, 28.12.2020 20:40


Health, 28.12.2020 20:40

Physics, 28.12.2020 20:40



English, 28.12.2020 20:40


Mathematics, 28.12.2020 20:40


Business, 28.12.2020 20:40


Mathematics, 28.12.2020 20:40


Business, 28.12.2020 20:40

English, 28.12.2020 20:40

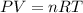
![25^oC=[25+273]K=298K](/tpl/images/0413/1404/df1f6.png)
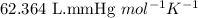

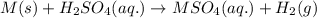
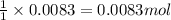 of metal
of metal