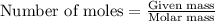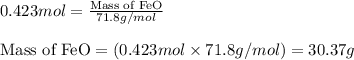Chemistry, 11.12.2019 05:31 nichelle2807
What mass of iron(ii) oxide must be used in the reaction given by the equation below to release 44.7 kj? 6feo(s) + o2(g) => 2fe3o4(s) δh° = -635 kj calculate your answer in g. enter it with two decimal places and no units.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3

Chemistry, 22.06.2019 09:00
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Chemistry, 23.06.2019 00:30
•hydration •dissociation •dissolving which one goes to which
Answers: 1

You know the right answer?
What mass of iron(ii) oxide must be used in the reaction given by the equation below to release 44.7...
Questions


English, 05.05.2020 18:00



Mathematics, 05.05.2020 18:00

Mathematics, 05.05.2020 18:00

English, 05.05.2020 18:00


Mathematics, 05.05.2020 18:00

Mathematics, 05.05.2020 18:00

English, 05.05.2020 18:00

Mathematics, 05.05.2020 18:00

Mathematics, 05.05.2020 18:00





English, 05.05.2020 18:01


Biology, 05.05.2020 18:01

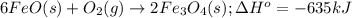
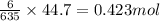 of iron (II) oxide is reacted.
of iron (II) oxide is reacted.