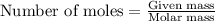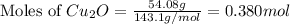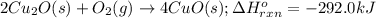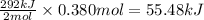Chemistry, 11.12.2019 05:31 Lydiac8715
The oxidation of copper(i) oxide, cu 2 o ( s ) , to copper(ii) oxide, cuo ( s ) , is an exothermic process. 2 cu 2 o ( s ) + o 2 ( g ) ⟶ 4 cuo ( s ) δ h ∘ rxn = − 292.0 kj mol calculate the energy released as heat when 54.08 g cu 2 o ( s ) undergo oxidation at constant pressure.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1


Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2

You know the right answer?
The oxidation of copper(i) oxide, cu 2 o ( s ) , to copper(ii) oxide, cuo ( s ) , is an exothermic p...
Questions


Physics, 14.01.2021 02:30

Computers and Technology, 14.01.2021 02:30



English, 14.01.2021 02:30


Mathematics, 14.01.2021 02:30

Mathematics, 14.01.2021 02:30

Mathematics, 14.01.2021 02:30


Arts, 14.01.2021 02:30



World Languages, 14.01.2021 02:30

Mathematics, 14.01.2021 02:30

Mathematics, 14.01.2021 02:30


English, 14.01.2021 02:30

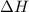 for the reaction will be -55.48 kJ
for the reaction will be -55.48 kJ