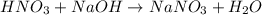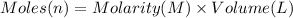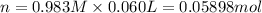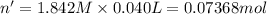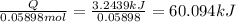Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Endeleev saw trends in the physical and chemical properties of elements when he organized them by
Answers: 2

Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1


Chemistry, 23.06.2019 07:30
Assignment directions: pick one of the following chemists and perform a bit of research on him/her. answer the following questions. alice hamilton rosalind franklin marie curie gertrude b. elion ada yonath henry cavendish robert boyle antoine lavoisier mario j. molina svante arrhenius
Answers: 1
You know the right answer?
In a calorimeter, you combine 60 ml of a 0.983 m hno3 solution with 40 ml of a 1.842 m naoh solution...
Questions


Mathematics, 27.07.2019 08:00



Mathematics, 27.07.2019 08:00



Mathematics, 27.07.2019 08:00

Computers and Technology, 27.07.2019 08:00

History, 27.07.2019 08:00

Health, 27.07.2019 08:00

Biology, 27.07.2019 08:00

History, 27.07.2019 08:00

Health, 27.07.2019 08:00

Mathematics, 27.07.2019 08:00


Mathematics, 27.07.2019 08:00

Mathematics, 27.07.2019 08:00


Mathematics, 27.07.2019 08:00