
A41.1 g sample of solid co2 (dry ice) is added to a container at a temperature of 100 k with a volume of 3.4 l. a. if the container is evacuated (all of the gas removed), sealed, and then allowed to warm to room temperature t = 298 k so that all of the solid co2 is converted to a gas, what is the pressure inside the container?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The first element on the periodic table of elements is carbon. a. true b. false
Answers: 2

Chemistry, 22.06.2019 03:00
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1


Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3
You know the right answer?
A41.1 g sample of solid co2 (dry ice) is added to a container at a temperature of 100 k with a volum...
Questions





Mathematics, 05.05.2020 04:09




Mathematics, 05.05.2020 04:09



Business, 05.05.2020 04:09


Chemistry, 05.05.2020 04:09


Mathematics, 05.05.2020 04:09

Spanish, 05.05.2020 04:10

Mathematics, 05.05.2020 04:10


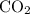 :
: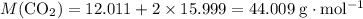 .
.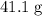 sample of
sample of 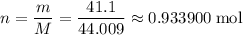 .
.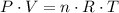 ,
, 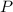 is the pressure inside the container.
is the pressure inside the container.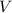 is the volume of the container.
is the volume of the container.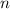 is the number of moles of particles (molecules, or atoms in case of noble gases) in the gas.
is the number of moles of particles (molecules, or atoms in case of noble gases) in the gas.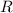 is the ideal gas constant.
is the ideal gas constant. 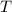 is the absolute temperature of the gas.
is the absolute temperature of the gas.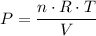 .
. .
.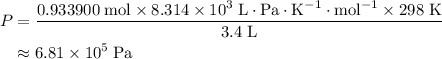 .
.

