
Chemistry, 11.12.2019 04:31 maguilarz2005
How much heat (in kj) is needed to convert 856 g of ice at −10.0°c to steam at 126.0°c? (the specific heats of ice, water, and steam are 2.03 j/g · °c, 4.184 j/g · °c, and 1.99 j/g · °c, respectively. the heat of fusion of water is 6.01 kj/mol, the heat of vaporization is 40.79 kj/mol.)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:40
What is the study of how matter and energy interact? a. biology b. physics c. planetary science d. chemistry
Answers: 1

Chemistry, 22.06.2019 03:00
Which of the dna typing techniques do you think you would choose if you had to analyze a dna sample? why?
Answers: 1

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1
You know the right answer?
How much heat (in kj) is needed to convert 856 g of ice at −10.0°c to steam at 126.0°c? (the specif...
Questions

English, 10.04.2020 14:27

English, 10.04.2020 14:27

Mathematics, 10.04.2020 14:27

Mathematics, 10.04.2020 14:27

Computers and Technology, 10.04.2020 14:27

Chemistry, 10.04.2020 14:27

Mathematics, 10.04.2020 14:28





Biology, 10.04.2020 15:23




Biology, 10.04.2020 15:23



Mathematics, 10.04.2020 15:23

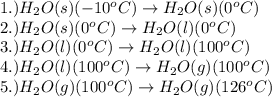
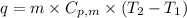 .......(1)
.......(1)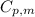 = specific heat capacity of medium
= specific heat capacity of medium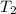 = final temperature
= final temperature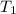 = initial temperature
= initial temperature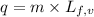 ......(2)
......(2)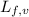 = latent heat of fusion or vaporization
= latent heat of fusion or vaporization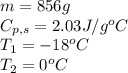
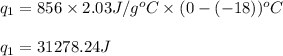
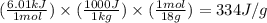

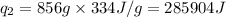
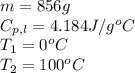
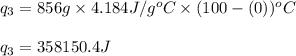
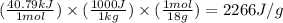
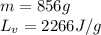
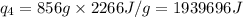
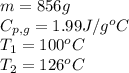
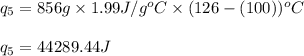
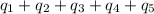
![[31278.24+285904+358150.4+1939696+44289.44]J=2659318.08J=2659.3kJ](/tpl/images/0412/9728/2c085.png)


