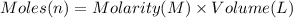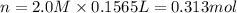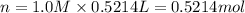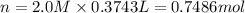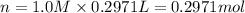Chemistry, 11.12.2019 02:31 savannahvargas512
Which of the following solutions contains the smallest number of ions? a. 156.5 ml of 2.0 m iron(iii) chloride b. 521.4 ml of 1.0 m lithium nitrate c. 374.3 ml of 2.0 m potassium hydroxide d. at least two of the above contain the smallest number of ions. e. 297.1 ml of 1.0 m sodium sulfate

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:40
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2

Chemistry, 22.06.2019 08:00
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 22.06.2019 21:30
What is happening when the water inside a kettle heats up and begins to boil
Answers: 1

You know the right answer?
Which of the following solutions contains the smallest number of ions? a. 156.5 ml of 2.0 m iron(ii...
Questions

Social Studies, 08.09.2021 19:00

Chemistry, 08.09.2021 19:00

Mathematics, 08.09.2021 19:00




History, 08.09.2021 19:00

Health, 08.09.2021 19:00

Mathematics, 08.09.2021 19:00

Social Studies, 08.09.2021 19:00




Social Studies, 08.09.2021 19:00

Mathematics, 08.09.2021 19:00





Social Studies, 08.09.2021 19:00