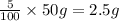Chemistry, 11.12.2019 02:31 sydthekid25
A1.0 g sample of hydrogen reacts completely with 19.0 g of fluorine to form a compound of hydrogen and fluorine. a. what is the percent by mass of each element in the compound? b. what mass of hydrogen would be present in a 50 g sample of this compound? c. justify your answer to b.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2



Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3
You know the right answer?
A1.0 g sample of hydrogen reacts completely with 19.0 g of fluorine to form a compound of hydrogen a...
Questions

Chemistry, 12.07.2019 16:00


Biology, 12.07.2019 16:00

Chemistry, 12.07.2019 16:00

Biology, 12.07.2019 16:00

Mathematics, 12.07.2019 16:00


Mathematics, 12.07.2019 16:00


Advanced Placement (AP), 12.07.2019 16:00


Mathematics, 12.07.2019 16:00

Advanced Placement (AP), 12.07.2019 16:00




Mathematics, 12.07.2019 16:00

Mathematics, 12.07.2019 16:00


Social Studies, 12.07.2019 16:00

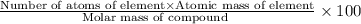
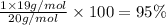
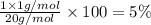
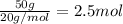
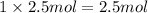 of hydrogen atom.
of hydrogen atom.