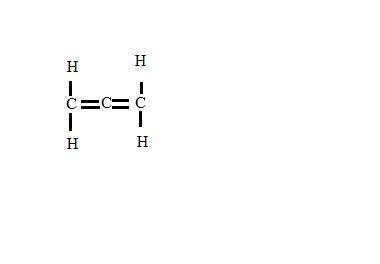Chemistry, 11.12.2019 01:31 puppylover72
Draw lewis structures for the following molecules. clearly label each center atom, then identify the molecular geometry for each center atom. select all the appropriate molecular geometries for the center atoms in the following molecule. c(ch2)2

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 14:30
Amixture that has two or more substances that are spread out evenly is called a. compound b. heterogeneous c. substance d. homogeneous
Answers: 1
You know the right answer?
Draw lewis structures for the following molecules. clearly label each center atom, then identify the...
Questions


Mathematics, 17.04.2021 18:40



English, 17.04.2021 18:40

Health, 17.04.2021 18:40

History, 17.04.2021 18:40

Mathematics, 17.04.2021 18:40

Mathematics, 17.04.2021 18:40


English, 17.04.2021 18:50

English, 17.04.2021 18:50

Business, 17.04.2021 18:50


Mathematics, 17.04.2021 18:50

Mathematics, 17.04.2021 18:50


Mathematics, 17.04.2021 18:50