
Chemistry, 11.12.2019 00:31 brendahndz8676
what is avogadro's number, and why is it useful? (3 points)
a. 2.033 x 1023; it is a conversion factor between moles and number of particles
b. 2.033 x 1023; it is a conversion factor between moles and mass
c. 6.022 x 1023; it is a conversion factor between moles and mass
d. 6.022 x 1023; it is a conversion factor between moles and number of particles

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Pbco3 –> pbo+ co2. how many liters of carbon dioxide gas is produced from the decomposition of 32 grams of lead (ll) carbonate?
Answers: 1

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 22.06.2019 23:00
What is a substance? a. a physical property of matter b. a chemical property of matter c. an element or compound that cannot be physically separated d. characteristics used to tell the difference between mixtures
Answers: 1

Chemistry, 23.06.2019 10:00
1.9 mol hcl and 3.9 mol naoh react according to the equation hcl + naoh −→ nacl + h2o . if the limiting reactant is hcl, calculate the amount of nacl formed.
Answers: 1
You know the right answer?
what is avogadro's number, and why is it useful? (3 points)
a. 2.033 x 1023; it is a co...
a. 2.033 x 1023; it is a co...
Questions

Mathematics, 22.06.2019 20:00

Health, 22.06.2019 20:00




English, 22.06.2019 20:00



Biology, 22.06.2019 20:00


Biology, 22.06.2019 20:00

Business, 22.06.2019 20:00


History, 22.06.2019 20:00

Biology, 22.06.2019 20:00





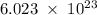 . It acts as a conversion factor between moles and the number of particles. Thus option D is correct.
. It acts as a conversion factor between moles and the number of particles. Thus option D is correct.

