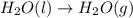Chemistry, 22.08.2019 16:40 sadieismichaeljackso
Regarding the vaporization of water, which of the following is true? a. a great deal of heat must be lost by water to form hydrogen bonds and allow molecules to move farther apart. b. a great deal of heat must be absorbed by water to break hydrogen bonds and allow molecules to move farther apart. c. vaporization of water is an exothermic process. d. water must lose a great deal of heat before it will vaporize.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1

Chemistry, 22.06.2019 05:30
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1

Chemistry, 23.06.2019 00:00
Total the mass on the syringe. record it in the correct row of the data table. kg done click and drag weights to change the pressure. click the syringe to zoom in and see the volume. intro
Answers: 3

Chemistry, 23.06.2019 02:00
Scientists are often interested in knowing the molar heat of combustion – the heat released during the combustion of one mole of a substance. use the periodic table to find molar masses. how many moles of ethanol are present in the sample?
Answers: 2
You know the right answer?
Regarding the vaporization of water, which of the following is true? a. a great deal of heat must b...
Questions

Chemistry, 28.02.2021 14:00


Mathematics, 28.02.2021 14:00

Mathematics, 28.02.2021 14:00


Mathematics, 28.02.2021 14:00

Mathematics, 28.02.2021 14:00

Spanish, 28.02.2021 14:00


Chemistry, 28.02.2021 14:00

Mathematics, 28.02.2021 14:00

Mathematics, 28.02.2021 14:00

Mathematics, 28.02.2021 14:00



Physics, 28.02.2021 14:00

English, 28.02.2021 14:00

Geography, 28.02.2021 14:00

Mathematics, 28.02.2021 14:00